

Founded in 2004, Hangzhou Clongene Biotech Co., Ltd. is a national high-tech enterprise specializing in providing Biological Raw Materials, diagnostic reagents, and CRO/CDMO services. 20 years of experience in the diagnostic industry, we have been committed to providing customers with high-quality products and professional technical services.
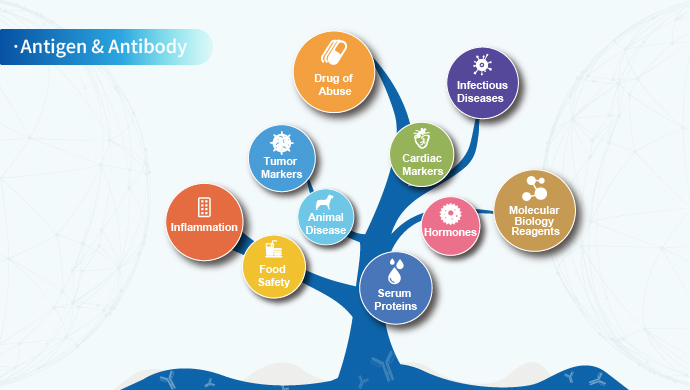
Based on the platform of protein expression and antibody preparation, Clongene has developed antigens, antibodies and molecular diagnostic enzymes in the fields of infectious diseases, drugs of abuse, fertility, tumor markers, inflammation, food safety and veterinary diseases. After preparation, purification and verification, the biological raw materials can be applied to immunochromatography, ELISA, chemiluminescence and PCR. We can also provide customized services for biological raw materials according to customer needs.
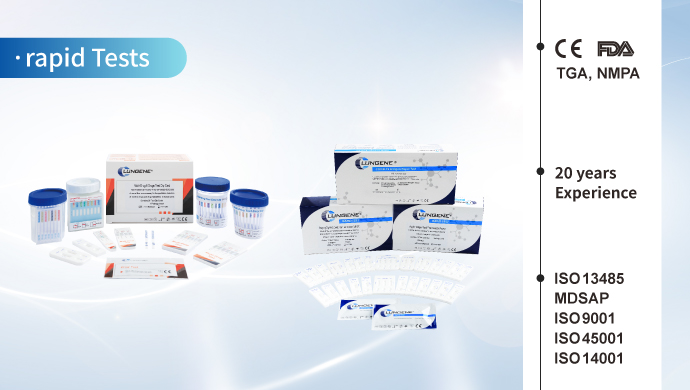
After 20 years of innovation and development, Clongene has developed colloidal gold/latex detection reagents, fluorescent immunoassay detection reagents, chemiluminescence detection reagents and PCR detection reagents. The main IVD reagents have obtained authoritative certifications including NMPA of China, FDA of US and CE of EU. The products were sold well in more than 100 countries and regions around the world.
Clongene is committed to bringing more value to companies and units around the world with a wealth of products and professional services, and to bring more opportunities for distributors and partners.
Based on the above-mentioned technology platforms, the products of Clongene Biotech mainly cover the fields of infectious diseases, drugs of abuse, fertility, inflammation, serum proteins, cardiac markers, tumor markers and animal disease.
| Biological Raw Materials | IVD Products |
Antibodies Antigens Enzymes | Colloidal gold immunoassay Fluorescent immunoassay Molecular diagnosis Chemiluminescence immunoassay |

Clongene Biotech is a one-stop solution enterprise integrating biological raw materials, detection reagents and instruments.
• High quality biological raw materials
• Multi and stable detection reagents
• Professional technical services

Clongene Biotech will continue to bring more value to companies and units around the world, and bring more opportunities to distributors and partners with rich products and professional services.
The main IVD reagents have obtained authoritative certifications including NMPA of China, FDA 510(k) clearances of US, TGA of Australia, CE of EU and some other certifications from different countries, which demonstrate the quality of our products are approved by related government institutions.
With independent innovation capabilities and rich technical experience, Clongene has obtained dozens of invention patents and utility model patents.
Clongene has been awarded as the innovation company in the key areas with national support, the Chinese People's Liberation Army material procurement supplier, Province-level R&D Center in Zhejiang, Patent Pilot Enterprise in Hangzhou and so on.
Clongene Biotech always takes product quality as the first priority. We have a 100,000-level GMP clean production area that meets GMP requirements to ensure product safety and quality.
Clongene Biotech has passed various system certifications such as ISO 13485, MDSAP, ISO 9001, ISO 45001, and ISO 14001 after 20 years of development.
