
On 21 January 2021, Member States unanimously agreed on a Council recommendation setting a common framework for the use of rapid antigen tests and the mutual recognition of COVID-19 test results across the EU.
The products need to meet the following requirements:
(a) Carry CE marking;
(b) Meet the minimum performance requirements of ≥ 90% sensitivity and ≥ 97% specificity; and
(c) Have been validated by at least one Member State as being appropriate for their use in the context of COVID-19, providing details on the methodology and results of such studies, such as the sample type used for validation, the setting in which the use of the test was assessed, and whether any difficulties occurred as regards the required sensitivity criteria or other performance elements.
The Health Security Committee agrees that, for rapid antigen test results to be mutually recognized, at least three Member States should be using a rapid antigen tests in practice.
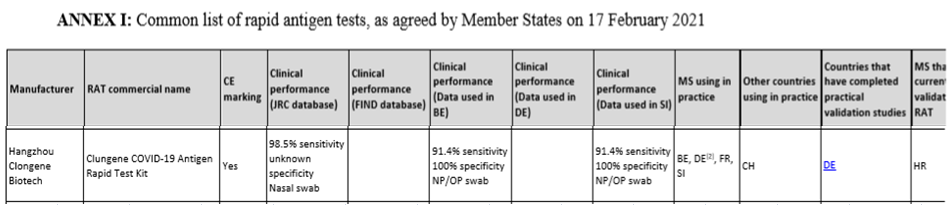
Clongene COVID-19 Antigen Rapid Test Kit with CE approved has become the main force of the anti-virus war in the EU, which also highlights the advantages of the product itself:
• easy to perform, but only for professional use.
• quick tests, enabling rapid implementation of infection control measures, including contact tracing.
• results are clearly visible.
• suitable for large-scale rapid screening.
• no equipment required.
Above all, Clongene will keep moving and protecting the great health of human!

About for Self-Testing
We, Hangzhou Clongene Biotech Co., Ltd. as the manufacturer for Clungene COVID-19 Antigen Rapid Test(Self-Testing) declare that we never has an exclusive distributor was authorized for German market