
“Clungene COVID-19 Antigen Rapid Test for self-testing” and “Clungene COVID-19 Antigen Rapid Test” manufactured by Clongene have obtained the TGA Certification in Australia on January 7, 2022. The certifications indicate that the Clungene COVID-19 Antigen Rapid Test products are approved to sold in the Australian market by the local government.
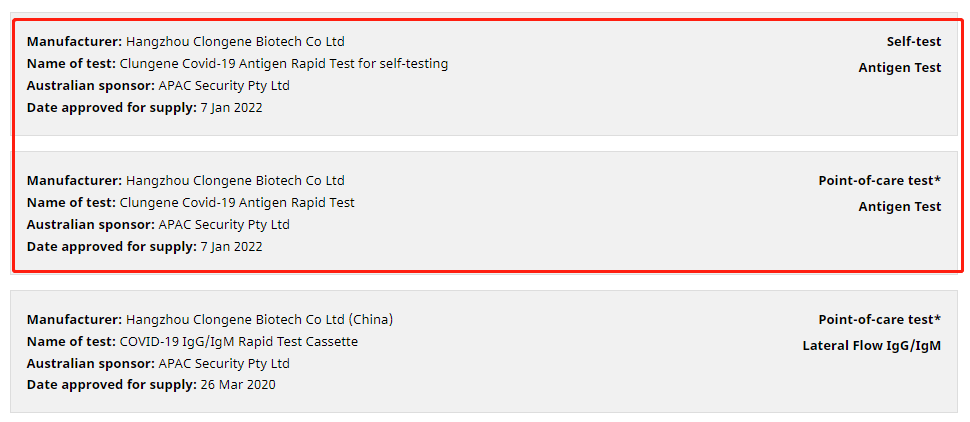
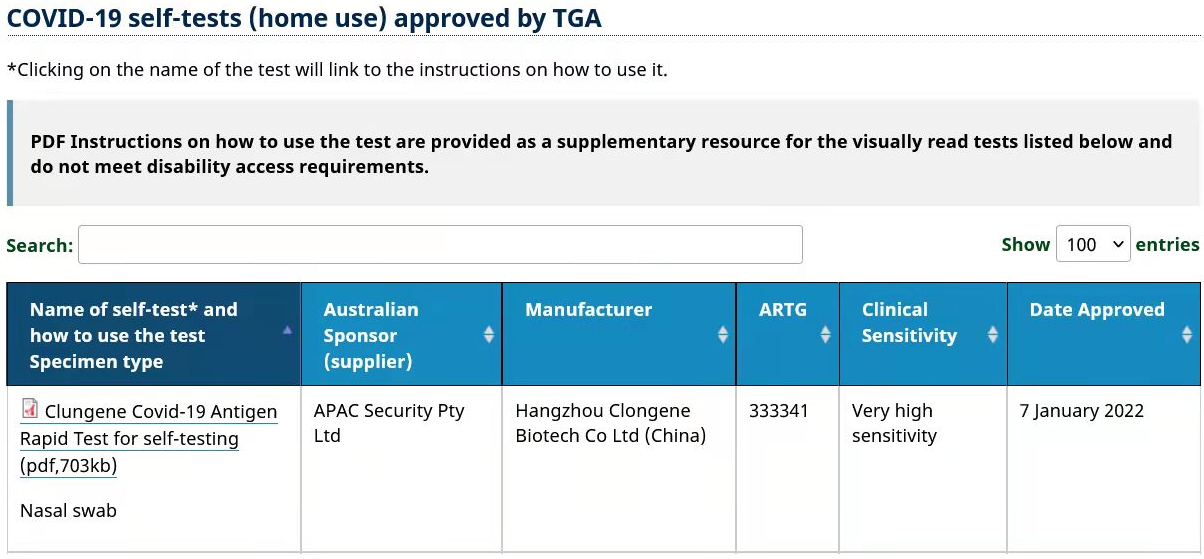
Australia is a well-known country with the strict management of drugs and higher requirements of market license. Therapeutic Goods Administration (TGA) is the federal drug regulatory agency under the Australian Department of Health. The products with the TGA certification are all evaluated by strict registration system in Australia.
Clungene COVID-19 Antigen Rapid Test products are easy to use without the equipment, and can read visible results at 15-20 minutes. As a international-renowned brand for rapid test of COVID-19, Clungene COVID-19 Antigen Rapid Test products are effectively qualified to conduct the rapid detection in most markets around the world.
Since the outbreak of the COVID-19, Clungene COVID-19 products have obtained the CE certification in EU, the TGA certification in Australia and some certifications from different countries, which demonstrate the quality of our products are approved by related government institutions. Also, our products have got good reputation from the main markets. Never stop moving forward! Clongene will keep to assist the prevention of the COVID-19 disease around the world and be an important force in the fight against the COVID-19.